What is Textile Surfactant?
The word Surfactant is a ‘Surface active agent’ contraction. The Surfactant compounds that reduce the interfacial surface tension between two liquids or between a liquid & a solid in textile material are called Textile Surface.
Classification of Surfactant:
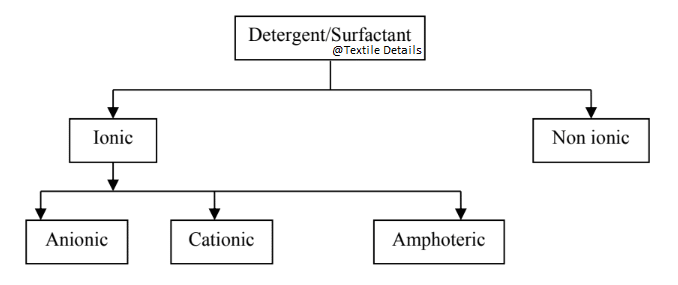
01. Anionic surfactant:
When the surfactant is ionized, the anion is the dominating ion in the solution, called an Anionic surfactant.
Example: Sodium Stearate is dissolved in water, anions CH3(CH2)16COO‾ is a large & dominating ion to Sodium ion.
Reaction: CH3(CH2)16COONa → CH3(CH2)16-COO– + Na+
02. Cationic surfactant:
When the surfactant is ionized, the cation is the dominating ion in the solution, called the Cationic surfactant.
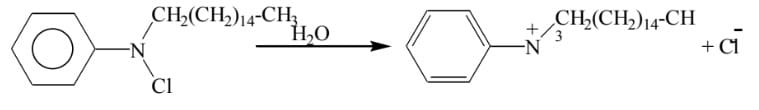
Example: Catyl pyridinium chloride is dissolved in water cation is large & dominating ion to chloride ion.
03. Nonionic surfactant:
Surfactants that don’t ionize when dissolved in water are called Nonionic surfactants. Example: Polyether formed by Stearic acid condensed with Ethylene oxide.
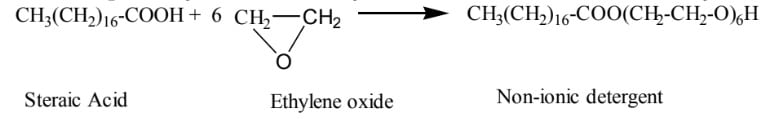
Example: Stearic Acid Ethylene oxide Non-ionic detergent
04. Amphoteric surfactant:
Surfactants, when dissolved in water, produce large segments carrying both anionic & cationic ions (zwitterion), which are called Amphoteric surfactants. Example: Amino carboxylic acids in which cationic amino and anionic carboxylic groups are present.

Unique characteristics of Amphoteric surfactant:
- In alkaline solution: PH>7, they behave like Anionic Detergent.
- In acidic solution: PH<7, they behave like Cationic detergent.
- In neutral solution: PH=7, they behave like Nonionic detergent.
- Amphoteric surface-active agents possess an affinity toward wool and cellulose fibers.
- They have lubricating properties.
Mechanism of surfactant:
- A surfactant molecule is divided into two parts when dissolved in water- The hydrophilic head & the Hydrophobic tail.
- Hydrophobic tails try to get away from the water & repelled by the water. Hydrophilic heads try to dissolve in water & dip at the water surface.
- As a result, the hydrophobic part reduces the surface tension & creates a force opposite to the inward pull on the water molecules. Hence the surface characteristics altered. For example, Sodium stearate is dissolved in water & divided into two segments- H2O
- The hydrocarbon chain (C17H35) is hydrophobic & the electronegative (COO–) ion is hydrophilic. So the hydrocarbon chains reduce the surface tension.
Why All soaps are Detergent, but all Detergent is not Soap?
Soap is a metallic salt of saturated/ unsaturated higher fatty acid (C9-C19).
Example: Sodium salt of stearic acid or Sodium stearate (C17H35COONa).
Which has head & tail end, oriented on the surface interface & reduces the surface tension is called Detergent.
So, Soap or any other compounds with those properties is referred to as detergents. For example, polyether ester has detergency power but is not a soap.
Difference between Soap and Detergent:
| Soap | Detergent |
| 1. Soap can give effective cleaning action to soft water only. | 1. Detergent can give to both soft and hard water. |
| 2. Soap is generally used as a cleaning agent for domestic purposes. | 2. It is used for textile purposes such as finishing, dyeing, laundry mills etc. |
| 3. All soaps are detergent. | 3. All detergents are not soap. |
| 4. Soap is costly. | 4. Detergent is cheap. |
| 5. Soap is Na or K salt of carboxylic acid. | 5. Detergent is any surface-active agent. |
| 6. No. of carbon 10 – 18. | 6. No. of carbon may vary. |
Other Textile auxiliaries in Wet Processing:
01. Acids/Alkalis
- To maintain PH.
- To fix dye on the fabric permanently.
- To develop the color of the fabric.
Example: Organic acid, Alkali KOH, NaOH, Na2CO3, potassium carbonate, sodium bicarbonate/acetate.
02. Swelling Agent
- To create big size holes in the fibers.
- Helps to swell the fiber structure.
- To reduce crystallinity.
- Help the easy penetration of dye molecules inside the fiber polymer.
Example: Di-Ethylene Glycol Diacetate (DEGDA), Polyethylene glycol, Phenols, etc.
03. Solvents/Dispersing Agents
- To get bright design by assisting dye penetration & fixation.
- To spread dye moles evenly in the solution.
- To prevent aggregation of dye molecules in the highly concentrated dye.
- To prevent precipitation & increase the solubility of the dyes.
Example: Alcohol, Acetone, Urea, Glycerine, Diethylene glycol, and so on.
04. Sequestering Agent
- Remove the hardness of the water.
- Increase solubility of dye.
- Sequester di-valent & tri-valent metal ions.
Example: Polyacrylate, Sugar acrylate, Hydrxy carboxylate.
05. Defoaming Agent
- To prevent foam generation during printing.
Example: Silicone, defoamers, sulfated oil, terminal KB, Emulsified pine oil.
06. Oxidizing Agent
- To develop the final color during steaming or subsequent after treatment.
- Assists with dye fixation.
Example: Sodium chlorate, Potassium chlorate, Sodium nitrate, Resist Salt Ammonium chloride, Ludigol, Na or K dichromate.
07. Reducing Agent
- To destroy color from the ground of fabric.
- Used for reduction of different dyes.
- To make the insoluble dyes soluble.
Example: Sodium hydrosulfite, Status chloride etc. Rongolite-C.
08. Salt
- Used as an electrolyte in dyeing.
- Enhance the exhaustion rate of the dyeing process.
Example: NaCl, NaOCl, NaNO2, Ca(OCl)Cl.
09. Brightening Agent
- To increase the apparent reflectance of the fabric & make it whiter & brighter.
Example: Optical Brightening Agent (OBA), Fluorescent Brightening Agent (FBA).
10. Miscellaneous Agent
| Anti-pilling agent: | To reduce pills in the fabric surface. |
| Hygroscopic agent: | Absorb moisture from the air. |
| Peroxide killer: | To destroy remaining peroxide content after bleaching. |
| Lubricating: | To minimize the formation of creases. |
| Leveling agent: | To uniform dyeing over the whole substance. |
| Retarding agent: | Retard or control the dye uptake inside the fiber. |
| Carrier: | Act as a catalyst to accelerate the dye penetration into the fiber. |
Reference: Lecture Sheet Name – Textile Surfactant & Textile Auxiliaries
TARIQUL ISLAM
Lecturer
Wet Processing Department
Textile Engineering College, Chittagong

Mahedi Hasan working as an Executive (Fabric Marketing) at Pengnuo Group. Graduated with B.Sc. in Textile Engineering. Before was a Top Rated content writer at Upwork, and Level 02 Seller at Fiverr, Level 02 Publisher at Ezoic. Very passionate about content writing, SEO practice, and fashion website designing. Highly Experienced fashion writer for the last 4+ years. Have extensive 7 years of experience in the wholesale clothing business.
