Our home planet boasts a unique feature, with water covering about 71% of its surface. It acts as a lifeblood, sustaining and nourishing every cell of plants, animals, and humans on earth. Furthermore, it participates intricately in the complex functions of the Earth’s climate, marking its unique and essential role in the complex processes of the planet. 98% of Earth’s water is salt water, and only 2% is freshwater; A little more than two-thirds is frozen.
Mainly, groundwater is the remaining unfrozen freshwater. Water Is the lifeline of the textile industry. According to the United Nations, The textile industry is one of the most water-intensive industries in the world, using large amounts of water in its production processes(1). The industry uses water in all stages of production, including dyeing, finishing, and washing clothes.
Table of Contents
Water and its uses in wet processing in the textile industry

- Pre-Treatment: Water removes impurities, oils, and natural substances from raw materials like cotton or wool before dyeing or further processing.
- Dyeing and Printing: Water is the medium for dyeing and printing textiles. It helps in the application of colorants onto fabrics.
- Finishing: Fabric finishing processes like washing, bleaching, and softening often involve water to achieve desired textures or appearances.
- Rinsing: Removing residual chemicals from the fabric after undergoing wet processing operations such as de-sizing, scouring, bleaching, and dyeing.
- Washing: Cleaning and modifying garments’ appearance, comfortability, and design. After each processing step, rinsing with water is essential to remove excess dyes, chemicals, or residues from the fabric.
Importance of water
Water consumption and discharge of wastewater are the two significant enterprises. The textile industry uses much water in its miscellaneous processes, particularly wet processing, similar to dyeing, pre-treatment, and printing.

Water is needed as a solvent of various dyestuffs and chemicals, and it’s used differently in washing or rinsing cataracts. Water consumption depends upon the operation styles, processes, dyestuffs, machines, and technology, which may vary from factory to factory and material composition.

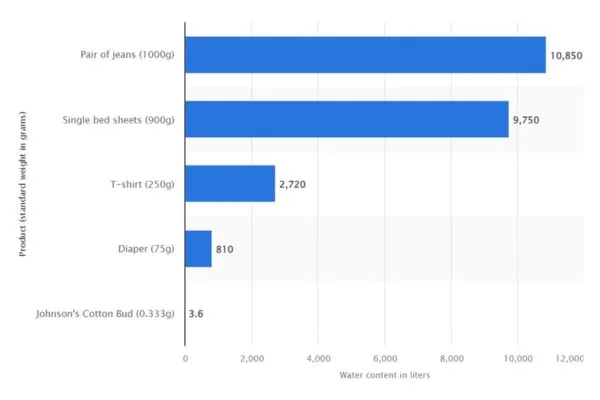
According to statistics, a pair of conventionally produced cotton jeans (1 kilogram) typically uses 10,850 liters of water. Single bed sheets (900 grams) will use 9,750 liters of water, and t-shirts (250 grams) will use 2,720 liters of water. (2) The fashion industry is one of the largest water consumers, and its water use is predicted to increase by 50% between 2007 and 2025 in developing countries and 18% in developed countries. (3)

Longer processing sequences, the processing of redundant dark colors, and reuse lead to redundant water consumption. And process optimization and the first-time production may save essential water.
Water footprint of select cotton textile products (in liters)
Sources of water:
Water with a high degree of purity is rarely obtainable from natural sources. The mineral constituents differ in quantity and relative proportions, depending on the source. Supplies of water may be broadly classified into four groups.
- Rainwater: Water that falls from the sky as rain.
- Surface water: The water found on the earth’s surface.
- Subsoil water: The water found in the soil’s pores.
- Deep well water: The water turned out from a wellbore extends at least 25 feet underground.
Impurities present in water
1. Suspended solid:
Suspended solids refer to small solid particles that remain in suspension in water as a colloid or due to the motion of the water. The particles make water appear cloudy or hazy. The practical range of the determination of filterable matter (TDS) is 10 mg/L to 150 000 µg/g.

The practical range of the Total Suspended Solids (nonfilterable particulate matter) is 4 to 20,000 mg/L. The practical range of the TDS – Total Dissolved Solid (filterable matter) is 10 mg/L to 150,000 µg/g. (4) TDS can vary depending on the specific soil and water conditions.
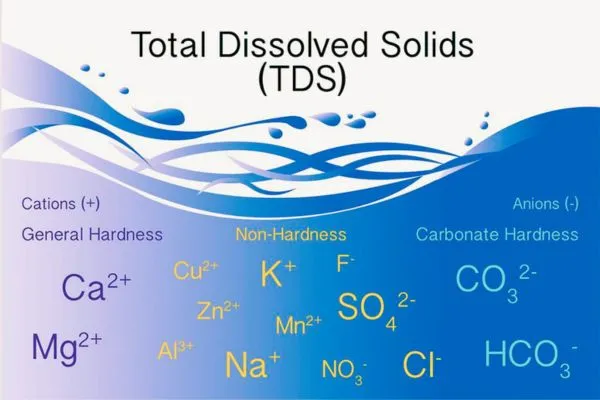
2. Dissolved mineral salts:
Calcium, magnesium, sodium, bicarbonate, sulfate, chloride, and nitrate are found in water as the principal ions. A few parts per million of iron or manganese may also be present. The exact concentration of these ions present in water can vary depending on the area’s geology and the water source.

3. Dissolved Gases:
Gases like carbon dioxide, oxygen, nitrogen, ammonia, and hydrogen sulfide are found present in varying quantities as dissolved in water.
4. Organic and coloring matters:
Dissolved Organic Matter (humus, peat, or decaying plant matter), Algae and Dinoflagellates, phytoplankton, Soil runoff, Iron and Manganese, and Natural Organic Matter (such as tannins or lignins) are found in water as organic and coloring matters.

5. Bacteria and other micro-organisms:
E. coli, Viruses, Algal toxins, Protozoans (such as Giardia and Cryptosporidium), cyanobacteria, algae, and tiny animals such as rotifers are found in water.
Hardness of water
Soft Water consists of a low concentration of calcium and magnesium salts. It gives foam with soap.
Example: Tap water, Drinking Water.
Hard Water: Hard water is due to the presence of high concentrations of calcium and magnesium salts (bicarbonates, sulfates, chloride) that are dissolved in water. It doesn’t form foam with soap. As calcium and magnesium salts react with soap to form insoluble organic salts, hard water does not easily create a lather with soap.
CaSO4 + 2RCOONa → (RCOO)2Ca ↓ + Na2SO4
MgSO4 + 2RCOONa → (RCOO)2 `Mg ↓ + Na2SO4
Classification of Hardness
1. Temporary Hardness
Temporary Hardness is due to the bicarbonate ion (HCO3-) of calcium and magnesium being present in the water. This type of hardness is called temporary hardness & When quick hard water is boiled, the bicarbonates decompose with the liberation of carbon dioxide and precipitation of the insoluble Carbonates, which are reformed, as indicated by the following equation:
Ca++ + 2HCO3 (boiling)→ CaCO3(s) + H2O(l) + CO2(g)
Formation: Rainwater contains dissolved carbon dioxide, making it acidic (carbonic acid). The acidic rainwater reacts with the limestone (calcium carbonate) and forms soluble calcium hydrogen carbonate.
2. Permanent Hardness
Permanent hardness is due to the presence of the ions Ca2+ Mg2+ in the form of Cl and SO42-.This type of hardness cannot be eliminated by boiling.
Formation: Rain water can run over rocks. Calcium sulfate (OR Gypsum) contains these ions (Ca2+, Mg2+). Once mixed in the water, hard water enters our streams and rivers. Thus, the permanent hardness of water is formed.
Methods of expressing hardness of water
The amount of calcium present in water expresses its hardness. Hardness expressed by:
1. PPM (Parts Per Million)
2. In degrees (Grains/ gallon)
3. Mg/L
Classification of water according to hardness
| DESCRIPTION | TOTAL HARDNESS |
| Very soft | 0-40 |
| Soft | 5-80 |
| Mild | 9-140 |
| Fairy hard | 15-180 |
| Hard | 19-300 |
| Very hard | >300 |
From the above types of water, soft water with a total hardness of 5-80 is suitable for dyeing. In other cases, like scouring, we may use hard water.
Impact of water hardness on textile processing
| Process | Problems |
| Desizing | Deactivates enzymes and makes it insolubilize some size materials like starch and PVA. |
| Scouring | Combine with soap and precipitate metal-organic acids. Produce yellowing of off-white shades, reducing cleaning efficiency and water absorption. |
| Bleaching | Decompose bleach baths |
| Mercerizing | Reduce absorbency and luster from insoluble metal oxides. |
| Dyeing | Break emulsions, change thickener efficiency and viscosity, and those problems indicated for dyeing. |
| Printing | Interfere with catalysts causes resins and other additives to become nonreactive, break emulsions, and deactivate soaps. |
| Finishing | Interfere with catalysts, causes resins and other additives to become nonreactive, break emulsions, and deactivate soaps. |
Different water-softening methods
- Soda lime process.
- Base exchange process (Permutit)
- Demineralization
- Soda alum
- Aeration
- Chelation on sequestration
Standard quality of dye house water
| MINIMUM STANDARD | PERMISSIBLE CONCENTRATION |
| Color | Colorless |
| Smell | Odorless |
| PH value | Nature (PH 7.8 ) |
| Dissolved solids | Less than 1 ml/L |
| Water hardness | Less than 50 dH |
| Solids deposits | Less than 50 mg/ L |
| Inorganic salt | Less than 500 mg/ L |
| Organic substances | Less than 20 mg/ L |
| Iron (Fe) | Less than 0.1 mg/ L |
| Copper (Cu) | Less than 0.005 mg/ L |
| Nitrate (NO3) | Less than 50 mg/ L |
| Nitrite (NO2) | Less than 5 mg/ L |
Consumption of water in wet processing
Water Consumption Numerous studies show that the daily water consumption of an average-sized textile mill in India, having a cloth production of about 8,000 kg/day, is about 1.6 million liters.
Conclusion
Water is important in textile processing, but its excessive use creates environmental challenges. Innovations in production methods aim to reduce the amount of water used to make garments. A balance between industrial growth and environmental responsibility is essential for the sustainability of the textile industry.
References:
- https://textilelearner.net/water-management-in-textile-industry/
- https://www.statista.com/statistics/561057/water-content-in-select-cotton-textile-products/
- https://www.theguardian.com/sustainable-business/sustainable-fashion-blog/2014/sep/04/10-things-to-know-water-impact-fashion-industry
- https://www.astm.org/d5907-18.html
- Water purification | PDF (slideshare.net)
- D5907 Standard Test Methods for Filterable Matter (Total Dissolved Solids) and Nonfilterable Matter (Total Suspended Solids) in Water (astm.org)
- Dyeing | PPT (slideshare.net)
